Pressure conversions
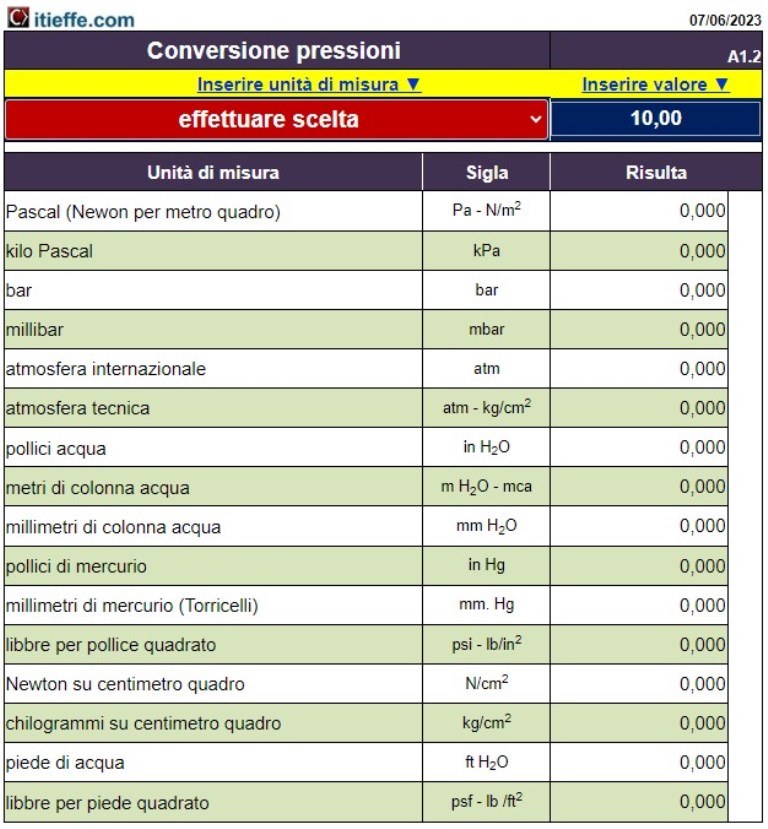
Simultaneous conversion of all values. By indicating one quantity you immediately have the value of all the others – Enter the known value in the corresponding cell and press enter
Pressure conversions
In the broad landscape of science and engineering, measuring and understanding pressure plays a crucial role. Pressure, defined as the force exerted on a surface per unit area, occurs in a variety of contexts, from the earth's atmosphere to industrial and medical applications. However, due to the different units of measurement used over time and in various industries, the need may arise to convert pressure values from one system to another in order to facilitate communication and understanding.
With this program we will see how simple it is to convert any pressure measurement unit directly into all the others, offering an overview of the results.
Pressure conversions
Pressure conversions
Online calculator for pressure unit conversion
By indicating the quantity of one quantity, one immediately has the value of all the others at the same time
Insights
Pressure
- The pressure and effort caused by a weight on a given surface.
- Pressure is a relationship between a weight and a surface.
Pressure = Weight / Surface (W / S)
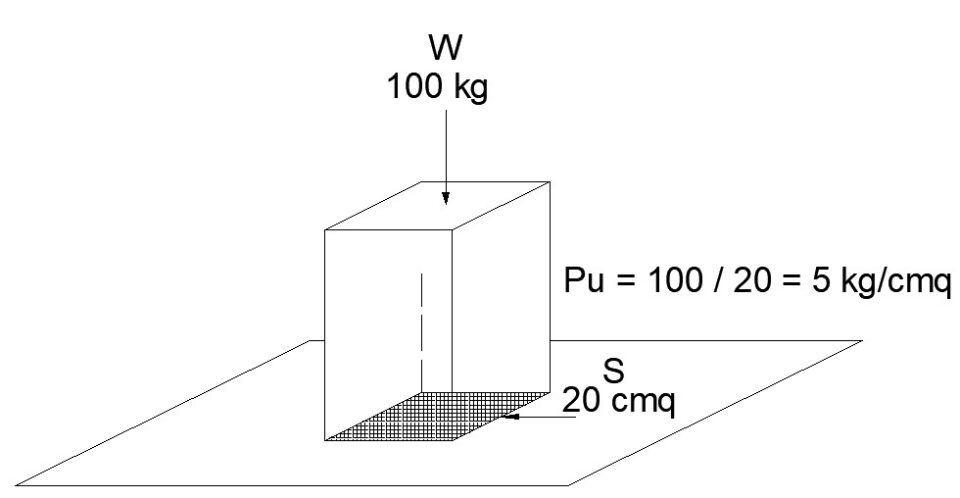
The same block of 100 kg placed on a surface S of 20 cm². exerts a pressure Pu of 5 kg / cm² on the surface, if with the same weight the surface S becomes 50 cm², the pressure Pu will be equal to 2 kg / cm².
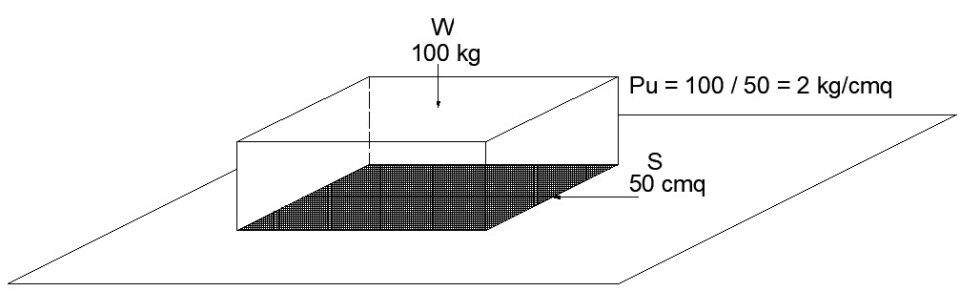
It is wrong to say that pressure is a weight: pressure is the effort produced by a weight, but on a given surface.
Atmospheric pressure
The air that surrounds us has a weight: with its weight it presses on the earth and on all things.
This weight is 1,29 kg / m³.
We do not notice it because the air pressure, on our body, is exerted both inside and outside the body itself and therefore is balanced.
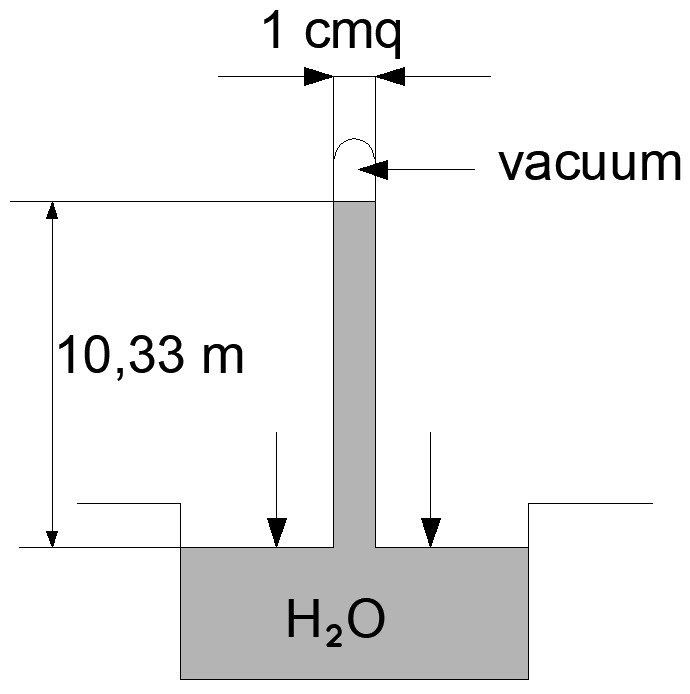
If in a tub of water (H.2O) we immerse a tube on which a vacuum has been made, as there is no air, a part of the atmospheric pressure will be missing in the tube, which will weigh only on the water of the tank.
The atmospheric pressure on the water in the tank, no longer balanced by that on the water in the pipe, pushes the water into the pipe itself, and the level of this water rises compared to that of the tank water to 10,33 meters.
This means that the atmospheric pressure represents a weight equal to a 10,33 m high water column.
We can ultimately say that atmospheric pressure is equal to the pressure of 1,033 kg per square centimeter.
Barometer (Evangelista Torricelli - 1608 - 1647)
If instead of water, we put mercury (Hg) in the tray, after vacuuming the tube immersed in the tray we would see the mercury rise in the tube up to a height of 76 cm.
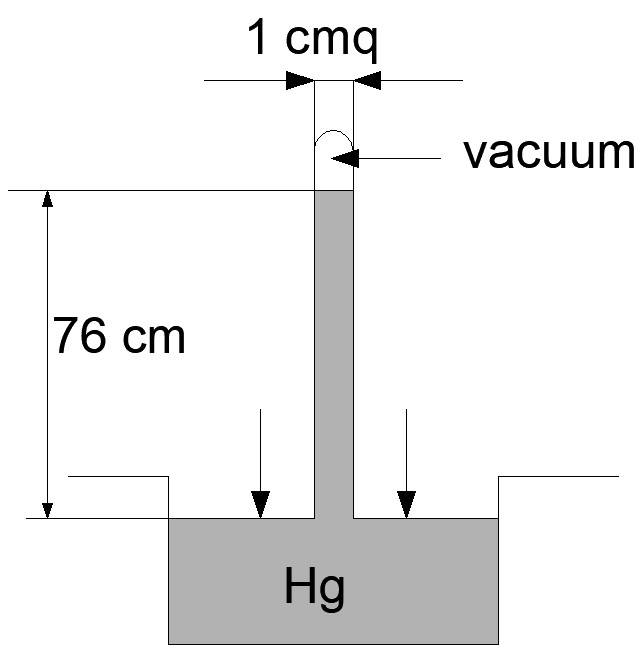
Mercury weighs 13,6 times more than water, so the height of the mercury column must be 13,6 times smaller than 10,33 m of water.
The mercury column must therefore be:
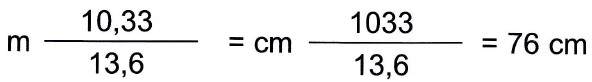
The atmospheric pressure is therefore also given by the weight of a 76 cm high mercury column.
All of this is correct when the experiment is performed at sea level.
But if we repeat the experiment in the mountains, we will see that the mercury column, instead of 76 cm will be, for example, 70 cm and the higher we repeat the experiment, the lower the height of the mercury column.
This means that the atmospheric pressure varies with the altitude and therefore the density of the air: density that is lower in the mountains (in fact we say that the air, in the mountains is finer, it is lighter.
The mercury tube thus described, graduated in centimeters or millimeters, would form the barometer an instrument that is to say the atmospheric pressure.
Pressure above atmospheric - Pressure gauges
Pressures higher than atmospheric are measured with an instrument called a manometer, taking as the basis of measurement the atmospheric pressure, also called "atmosphere".
If, for example, in a tank there is a fluid at a pressure of 3 times the atmospheric pressure (pressure, that is, capable of lifting a column of water at m 3 x 10,33 = m 30,99) we will say that the pressure of the fluid is 3,09 atmospheres.
That is, it was agreed to call the atmospheric pressure with the word "atmosphere" and to measure the pressure in atmospheres.
The atmosphere is therefore the stress that atmospheric pressure exerts on the bodies, and is equal to 1,033 kg per square centimeter.
To simplify the practical calculations, the atmosphere is considered equal not to 1,033 kg but to 1 kg per square centimeter, abbreviating 1 kg / cm². ultimately when we talk about pressure, we can say that the atmosphere is the pressure of 1 kg / cm² which would correspond to a 10 m column of water.
Pressure gauges are those devices that are used to measure the pressure of a liquid, a gas, a vapor.
They are graduated in kg / cm².
Absolute pressure and actual pressure
If the pressure gauge of a boiler shows 2 it means that the water it contains has a pressure of 2 kg / cm² and is indicated by the abbreviation ATE.
But by measuring in this way the pressure of the water contained in the boiler we do not take into account the atmospheric pressure. This pressure, we have said, is exerted on all existing bodies on earth, therefore also on our boiler and on its contained water.
Wanting, therefore, to take into account the atmospheric pressure, the total pressure or, as they say, ABSOLUTE of the water is not 2 but 2 plus the atmospheric pressure, i.e. 2 + 1 = 3 and is indicated with the abbreviation ATA ( absolute atmospheres).
Therefore the actual pressure is the pressure actually marked by the manometer; absolute is that marked by the pressure gauge increased by 1 unit. In the above case we will write that the water in that boiler has a pressure of 2 kg / cm² effective (ie 2 ATE) or has 3 kg / cm², absolute (ie 3 ATA).
Instructions

How to proceed:
1 – select the unit of measurement
2 – enter the value
press enter or click inside the program.
3 – The conversions made will be displayed immediately
4 – The arrow indicates the chosen unit of measurement
Other free programs of the same kind offered by itieffe ▼
Pressure conversions
The program below is free to use.
To access the reserved version (see below), full page and without advertising, you must be registered.
You can register now by clicking HERE
