Fuel characteristics

Solid - liquid - gaseous fuel characteristics
Energy is at the center of our lives and economies, and a significant portion of this energy comes from solid, liquid and gaseous fuels. These fuels constitute a vital resource for a wide range of applications, from electricity production to industry, from transport to home heating. This paper was created by Itieffe to explore in detail the characteristics of these fuels, offering an in-depth view of how they are produced, used and what impacts they have on our world.
This premise will give you a clear idea of what you can expect from this paper, which will address the main types of solid, liquid and gaseous fuels, explaining their properties and applications.
Understanding the characteristics of fuels is essential to efficiently exploit these energy resources and to sustainably manage the environmental impact of their production and combustion. This knowledge is essential for engineers, designers, plant operators and energy professionals.
This paper will cover a range of topics related to solid, liquid and gaseous fuels:
- Definition and types of fuels in each category.
- Specific weight of fuel in each category.
- Its viscosity and sulfur content.
- The lower calorific value (kcal/h) of different fuels.
- The volume of combustion air and the volume of combustion products.
Knowledge of the characteristics of fuels is crucial for the future of energy and for addressing the environmental challenges linked to the production and use of fossil and renewable fuels. This paper will help you better understand the role of solid, liquid and gaseous fuels in our society and in the transition towards a more sustainable future.
We are happy to share this knowledge with you and explore the world of fuels and their characteristics together. Your understanding of these concepts is critical to contributing to a more efficient and sustainable energy future.
Solid - liquid - gaseous fuel characteristics
In relation to the physical state in which they occur, fuels are classified into solid, liquid and gaseous. Another important distinction of fuels is between "natural" and "derivatives", in relation to the conditions in which they are used: natural (eg: methane gas) if they are used as they are found in nature or derivatives (eg: petrol , LPG) if they are supplied as products of transformation of natural fuels or of particular industrial processes. Or:
- natural solids: e.g. wood, fossil coals (peat, anthracite, lithanthrac), etc.
- artificial solids: eg. coke and charcoal
- natural liquids: eg. oil and bitumen
- artificial liquids: eg. gasoline and other petroleum distillates, alcohol, etc.
- natural gases: eg. methane
- artificial gases: eg. illuminating gas, blast furnace gas, hydrogen (water gas), acetyl
Liquids and solids being in a state of aggregation different from the gaseous reactant, develop heterogeneous combustions for which close contact between the chemically active parts is required; in the case of gases and liquid vapors, on the other hand, since they are homogeneous systems, the process takes place through the mixing of reactants.
source Wikipedia
Solid fuels
Solid fuels are fuels that occur in a solid state.
They are divided into:
- natural
- artificial
Among the natural fuels, fossil fuels constitute a group of their own, although obviously not isolated.
Solid fossil fuels are all solid combustible material formed over the course of geological eras: coal and oil shale belong to them.
Coals and shales can have variable composition and characteristics within a very wide range.
There is no single theory that can fully explain the formation of all known types of carbon. The age of the deposit can vary by hundreds of millions of years; a common feature of all fossil fuels is that they consist mainly of carbon and hydrogen.
Peat can also be considered fossil fuels, of relatively recent formation.
The most important non-fossil solid natural fuel is wood, from which fossil fuels are derived.
Artificial solid fuels must be considered, in addition to charcoal, obtained by heating wood to 500-600 ° C in the absence of air, coke and semi-coke, coming from the coking processes of fossil coal and oil.
Finally, among solid fuels we also mention metal-based ones (lithium, beryllium, boron, sodium, magnesium and aluminum) which exploit the high heat of combination with oxygen.
Main characteristics of solid fuels.
The main characteristic of solid fuels (as well as that of other types of fuels) is the upper and lower calorific value (pcs - pci) which is measured in kilocalories or joules per unit of weight (kg) or volume (liter or m3).
Other important features are:
- the sulfur content; a low value of this parameter is preferable in order to avoid the formation of sulfur oxides (atmospheric pollutants responsible for acid rain) during combustion.
- the residue in ashes.
Liquid fuels
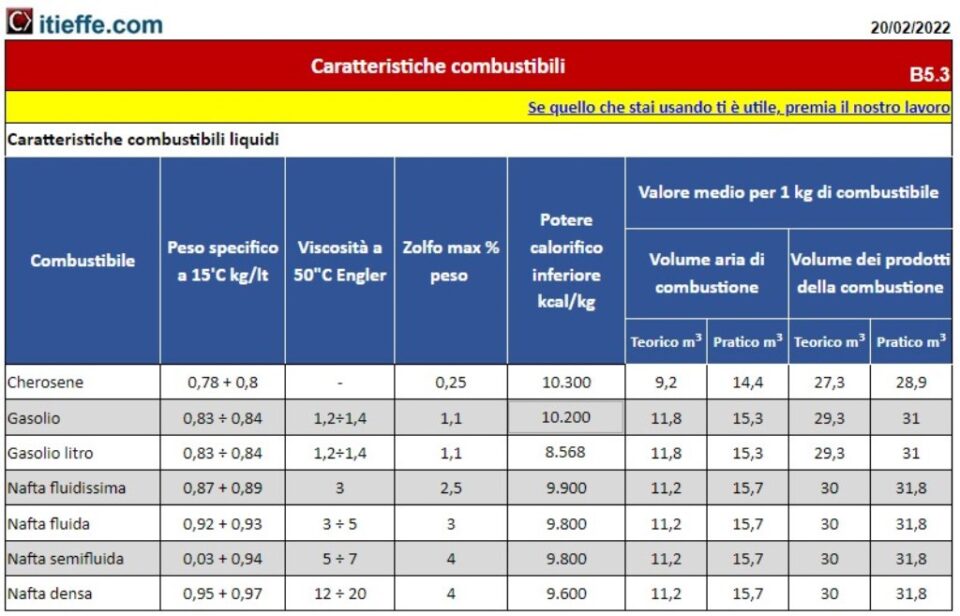
Liquid fuels are fuels that occur in a liquid state.
Natural liquid fuels consist exclusively of petroleum, asphaltic bitumen and tar sands in which the combustible part is actually exclusively liquid.
Liquid fuels are then mainly those produced in oil fractionation operations and in the various treatments to which the individual fractions or the crude oil are subjected (for example: petrol, fuel oil, kerosene, etc.).
Main characteristics of liquid fuels
The main characteristic of liquid fuels (as well as that of other types of fuels) is the upper and lower calorific value (pcs - pci) which is measured in kilocalories or in joules per unit of weight (kg) or volume (liter).
Other important characteristics are: the sulfur content; a low value of this parameter is preferable in order to avoid the formation of sulfur oxides (atmospheric pollutants responsible for acid rain) during combustion
As far as liquid fuels are concerned, the ease of storage and transport is interesting, making them preferable to solid fuels.
Gaseous fuels
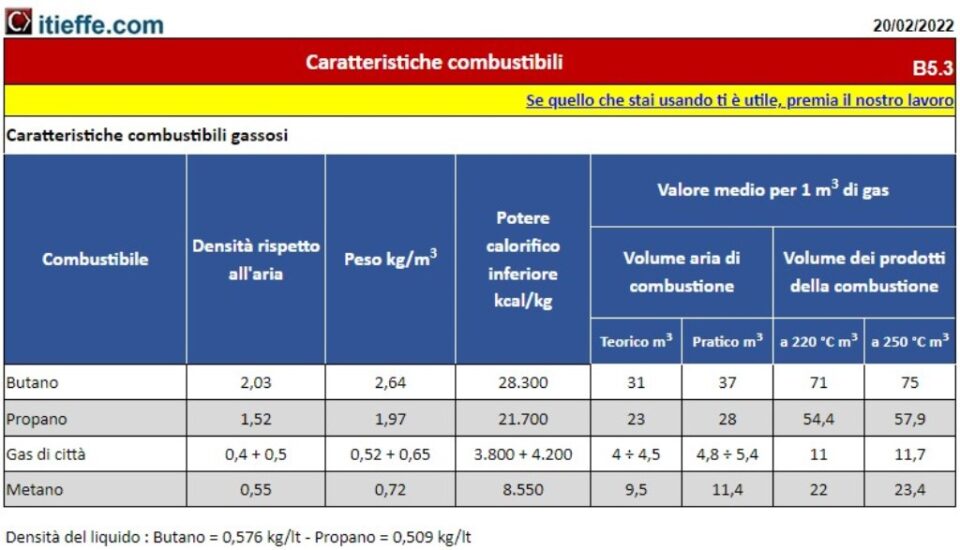
Gaseous fuels are hydrocarbons composed of carbon and hydrogen atoms. Depending on the molecular structure of the atoms, i.e. their number and arrangement, the gas acquires the property of fuel. Gaseous fuels are characterized by a flame that is not excessively hot but bright. The gas takes on the properties of fuel only when it is mixed with oxygen in the air within an adequate concentration ratio.
There are different types of gaseous fuels. The main ones are the illuminated gas produced by the distillation of coal, the natural gas present in the subsoil or produced by livestock activities, the LPG gas obtained from the liquefaction of light hydrocarbons, the gas generator gas and the blast furnace gas.
Characteristics of gaseous fuels Gaseous fuels are odorless and colorless. For this reason they are added with substances capable of adding an unpleasant odor (eg sulfur) in order to allow users to realize any gas leaks from the pipes or devices used. Solid fuels are toxic, for example carbon monoxide, and very dangerous for human safety and health. Furthermore, gas combustion can release various types of combustion residues into the air (nitrogen, water, etc.).
Applications of gaseous fuels In the past, gaseous fuels were used for street or domestic lighting (illuminating gas). Each lamppost was equipped with a spout, from which the gas came out, and a flame. When the gas came into contact with the flame and with the air, it produced combustion and therefore light. Urban lighting with gas lamps and gas lamps became obsolete with the spread of electricity which has practically replaced the illuminating gas in almost every city in the world. Today solid fuels are used as fuel for domestic heating, for cooking (gas cookers) and for the production of electricity in gas or turbo-gas power plants.
Other free programs of the same kind offered by itieffe ▼
- Heating - Plumbing
- Pipelines
- Heating tables
- pumps
- Heating drawing diagrams
- Domestic hot water
- Combustible gases
Solid - liquid - gaseous fuel characteristics
The program below is free to use.
To access the reserved version (see below), full page and without advertising, you must be registered.
You can register now by clicking HERE
