Temperature and latent heat of fusion
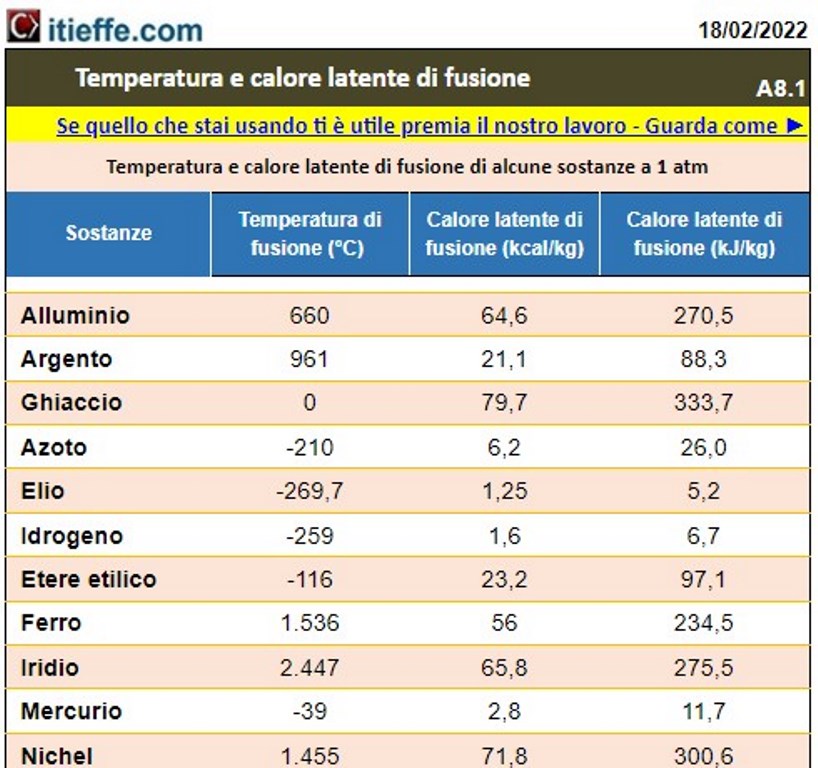
Melting temperatures – latent heats of fusion
This paper created by Itieffe is a document aimed at exploring the fundamentals of thermodynamics and providing a clear understanding of the concepts of temperature and latent heat of fusion, essential concepts in the fields of physics and engineering, as they provide a solid basis for understanding how matter changes phase and how heat is transferred between bodies.
Temperature is a fundamental quantity that measures the kinetic energy of particles in a system. In this paper, the basics of temperature, its measurement scale and how temperature variations influence the physical state of a substance will be addressed.
Latent heat of fusion is another key concept that will be covered in detail. This is the amount of heat necessary to make a substance pass from the solid phase to the liquid phase (fusion) or vice versa (solidification) without the temperature changing.
A fundamental phenomenon for understanding the freezing and melting processes of substances such as water, but it also has applications in various disciplines, from physics to chemistry to engineering.
Understanding temperature and the latent heat of fusion is fundamental in many scientific and engineering disciplines, and is essential for solving problems and designing systems involving phase changes and heat transfer.
This paper offers a comprehensive overview of these topics, serving as an invaluable guide for anyone wishing to deepen their understanding of thermodynamics and the physical principles that govern the world around us.
Latent heat of fusion e temperature
Latent heat of fusion is the amount of energy needed in KJ or kcal, to completely melt 1 kilogram of substance at the melting temperature.
During solidification, a liquid gives off heat. The latent heat of solidification represents the heat that the unit mass of liquid provides during the change of state. The latent heat of solidification is numerically equal to the latent heat of fusion.
TLatent heat of fusion e temperature
The program below is free to use.
To access the reserved version (see below), full page and without advertising, you must be registered.
You can register now by clicking HERE
