Lower calorific value (PCI)
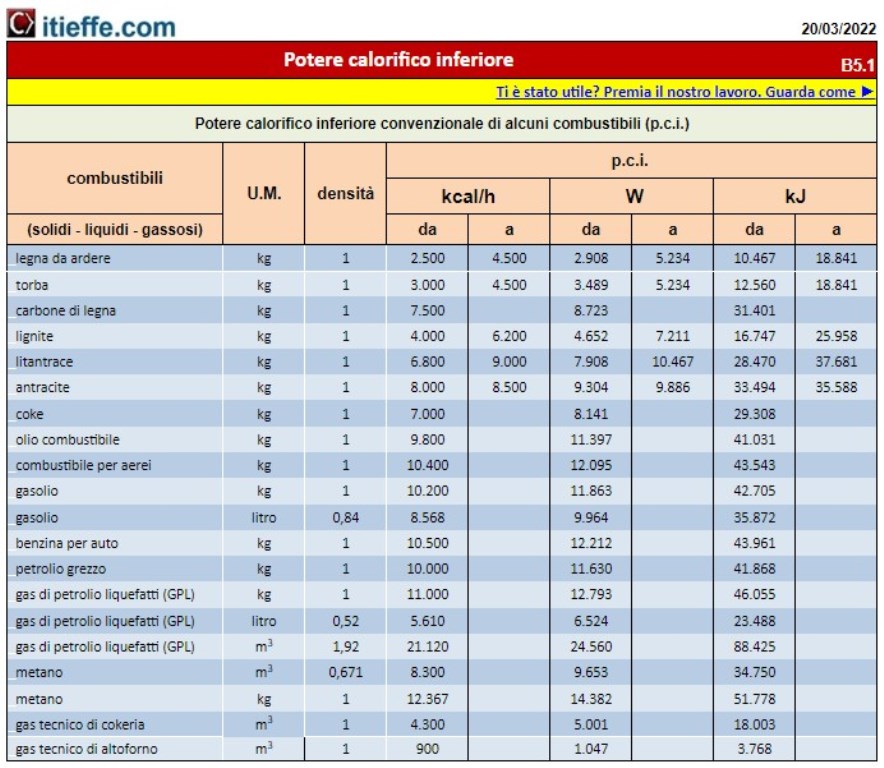
Lower calorific value (pci) of some fuels
In the field of energy and thermodynamics, the concept of “Lower Calorific Value” (Lower Calorific Value) is a fundamental pillar that permeates a wide range of sectors and applications. This essay was created by Itieffe to delve deeper into the topic, explaining its importance, its calculations and its practical implications. During this preface, we will offer you a preview of the topics that will be covered in this paper.
The Lower Calorific Value is a crucial quantity in the field of combustion and energy. It represents the amount of heat released during the combustion of a specific fuel, and is a key indicator of the efficiency of a combustion process. Its understanding is fundamental for the sizing of energy systems, the evaluation of energy resources and the optimization of processes.
Understanding the dictates of this guide is fundamental for energy engineering, industrial efficiency and the management of energy resources. This paper will help you fully grasp the importance of PCI and use it effectively in your applications and projects. In an age where sustainability and energy efficiency are global priorities, PCI plays a significant role in our transition to a more sustainable future.
We are happy to share this knowledge with you and explore this world together. By understanding this concept, you will be better equipped to meet challenges and exploit opportunities in energy and combustion.
The Lower Calorific Value (PCI)
It is divided into "Superior" and "Inferior"
PCS – The gross calorific value takes into account the latent heat of water vaporization generated during combustion:
it is the amount of heat which becomes available due to the effect of complete combustion at constant pressure of the unit mass of the fuel, when the combustion products are brought back to the initial temperature of the fuel and the oxidizing agent.
The determination of the calorific value can be obtained approximately with the calculation, based on the elemental analysis of the fuel, or directly through the use of suitable calorimetric instruments.
PCI – The lower calorific value, considered by this section, does not take into account the latent heat of vaporization of water generated during combustion.
In normal combustion, the combustion products are released at a higher temperature than the reference temperature of the fuel. Thus, a part of the theoretically available heat is lost due to the heating of the fumes and, above all, due to the vaporisation of the water produced by the combustion.
Conventionally, the lower calorific value is defined as the upper calorific value less the heat of condensation of the water vapor during combustion.
This is the value usually referred to when talking about the calorific value of a fuel and the efficiency of a thermal engine.
In modern condensing boilers it is possible to recover part of the latent heat of the water vapour. This fact makes it possible to obtain, from a kg of fuel, a quantity of heat greater than the lower calorific value, therefore, with nominal efficiency equal to 100%, even if a part of the theoretically available heat (higher calorific value) continues to be dispersed with the you smoke.
Taken from Wikipedia
Lower calorific value (PCI)
The main characteristic of a fuel is its calorific value.
This represents the amount of heat developed in the combustion reaction under predefined standard conditions.
It is generally measured in kcal / kg for solids and liquids, while for gases it is expressed as kcal / m³
In most fuels, which contain hydrogen, there is a higher calorific value (which includes the condensation heat of the water vapor that is formed in combustion) and a lower calorific value (which does not consider this heat).
Methane, the most common gas for heating, has a heating power of about 10 kW per cubic meter (9,6, to be precise): this means that if we keep the boiler on for an hour, to make 24 kW we will need about 2,5 cubic meters of methane.
How many kg of LPG in a cubic metre? – In normal temperature and pressure conditions of use, LPG for domestic use in the liquid state weighs approximately 0,52 kg/litre, while in the form of gas, LPG has a specific weight of approximately 2 kg/m³.
Other free programs of the same kind offered by itieffe ▼
- Heating - Plumbing
- Pipelines
- Heating tables
- pumps
- Heating drawing diagrams
- Domestic hot water
- Combustible gases
Lower calorific value (PCI)
The program below is free to use.
To access the reserved version (see below), full page and without advertising, you must be registered.
You can register now by clicking HERE
