Refrigerant gas
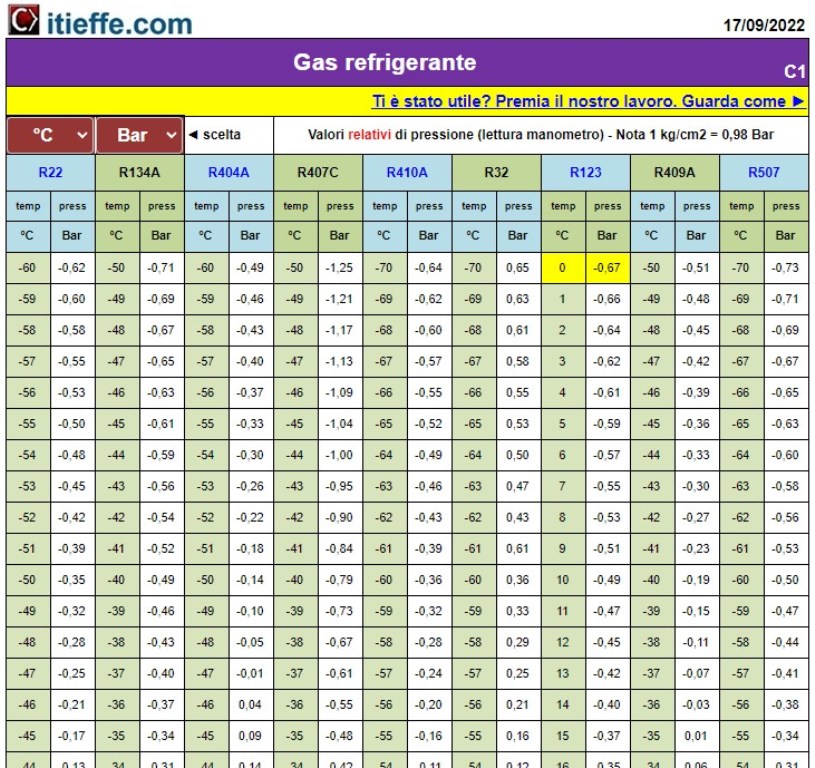
Characteristic values of the various types of refrigerant gases. Values also in degrees Fahrenheit and PSI ((pound square inch)
The objective of this guide created by Itieffe is to provide a complete overview of some characteristic values of the different types of refrigerant gases commonly used in the refrigeration and air conditioning industry. Understanding these values is critical to selecting and using refrigerant gases safely and efficiently. The guide will cover the following categories of refrigerant gases:
- Pure Gases: Including simple refrigerant gases such as R-134a and R-410A.
- Zeotropic Blends: Like R-407C and R-404A.
- Azeotropic Mixtures: Like R-507A and R-410A.
- Natural and Alternative Gases: Like ammonia (NH3) and propane (R-290).
Characteristic Values to Consider:
For each category of refrigerant gas, the following characteristic values were analyzed and indicated:
- Temperature values: saturation temperature.
- Saturation pressure: the pressure at which the gas is in equilibrium between the liquid and gaseous states at a given temperature.
For all types of gas, the indication in Anglo-Saxon units of measurement is also shown.
Conclusions:
Understanding the characteristic values of refrigerant gases is essential for the effective design, use and maintenance of refrigeration and air conditioning systems. The selection of the appropriate refrigerant gas depends on a number of factors, including energy efficiency, environmental impact and application specifics.
It is important to note that the industry is evolving towards the adoption of low GWP refrigerant gases and greener alternatives to reduce the overall environmental impact. Therefore, it is essential to stay up to date on refrigerant gas trends and regulations to ensure the sustainability and compliance of your operations.
Refrigerant gas
Characteristic values of the various types of refrigerant gas.
Values also expressed in degrees Fahrenheit (° F) and in PSI ((pound square inch)
A refrigerant fluid (or simply refrigerant) is the operating fluid of a refrigeration cycle. It has the task of moving heat according to the transmission of heat from a hot source to a cold source and this transfer can take place through latent heat exchange (evaporation, condensation) and / or through sensible heat exchange (heating, cooling).
The refrigerant should have:
- high density, both in the gaseous and liquid phase;
- high enthalpy of vaporization;
- high thermal capacity;
- high stability in the conditions of use.
Fluids for refrigeration cycles
The fluids can be natural (ammonia, carbon dioxide, propane, etc.) or artificial (usually called Freon).
Both artificial and natural fluids are used in vapor compression cycle, for the absorption cycle ammonia or lithium bromide (absorption) and silicagel or zeolites (adsorption) are used.
The most important characteristics of a refrigerant fluid to be used in a vapor compression cycle are due to the need to reduce the work of the compressor.
CFC molecules were the first to be used in compression refrigeration cycles, as the replacement of a hydrogen atom with a fluorine atom is a simple operation that causes an increase in density, and generally causes an increase in density. enthalpy of evaporation and an increase in the boiling temperature.
In addition to pure refrigerants, there are also multi-component mixtures grouped in the R400 series (non-azeotropic mixtures, which see the temperature change in the phase transformation at constant pressure) and in the R500 series (mixtures with azeotrope, with behavior equal to that of a fluid pure).
Other refrigerant fluids are: R170 ethane, R290 propane, R600 butane, R600a isobutane, R-610 diethyl ether, R717 ammonia, R744 carbon dioxide, R-1150 ethene (ethylene), R-1270 propene (propylene).
From Wikipedia, the free encyclopedia.
Storage and transport of refrigerant fluids
The refrigerant fluids intended for the air conditioning and refrigeration market are stored in special cylinders of various sizes. The name on the label corresponds to the ASHRAE code of the product and the price is based on weight. The pressure varies between 30÷60 bar and in these conditions the refrigerants are in liquid form and the filling is never complete because an equilibrium is formed with the vapor phase which thickens in the head space of the cylinder.
For non-azeotropic mixtures, the composition of the gaseous phase is different from that of the liquid and therefore taking the gaseous phase or the liquid phase from the container means obtaining two mixtures with different concentrations and properties. In cylinders with a single valve, the liquid is withdrawn by turning the cylinder itself upside down, but newly built cylinders are equipped with a double tap: one for the gas and one for the liquid connected to a dip tube near the bottom.
Other free programs of the same kind offered by itieffe ▼
The program / paper shown below is free to use.
To access the reserved version (see below), full page and without advertising, you must be registered.
You can register now by clicking HERE
