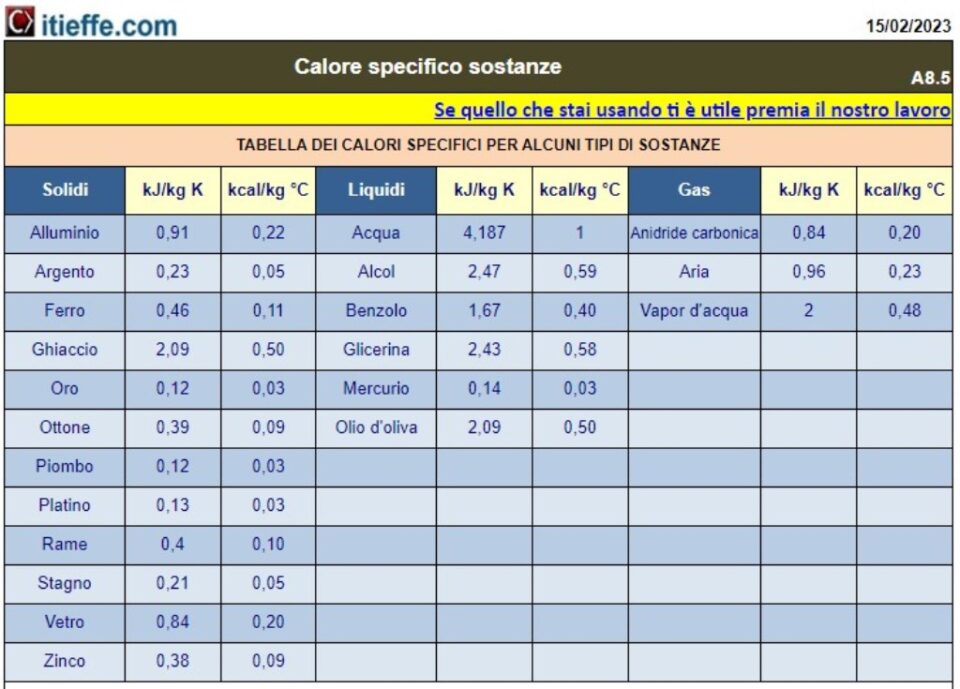
Specific heat table of substances
This paper created by Itieffe represents a fundamental resource in the field of thermodynamics and materials science, offering a detailed overview of the thermal properties of different substances.
This document aims to clearly and concisely explain the concept of specific heat and provide meaningful data on a variety of materials, helping students, scientists and engineers understand how different substances respond to changes in temperature and provide essential information for the design of thermal and energy systems.
In this introduction, we wish to underline the importance of specific heat as a fundamental parameter for the thermal characterization of materials. Specific heat represents the amount of thermal energy needed to raise the temperature of a given amount of matter by a certain amount of degrees Celsius or Kelvin.
This quantity is crucial for a wide range of applications, from the design of electric motors and cooling circuits to the calculation of energy needs in industrial processes.
The main features and topics addressed in this paper include:
- Definition of specific heat: we will explain in detail what specific heat represents and how it is measured experimentally.
- Unit of measure: we will illustrate the units of measurement of specific heat and how they are used in thermodynamic formulas.
- Specific heat of different substances: we will provide a list of specific heats for a wide range of common materials, including metals, liquids, gases and others.
The main objective of this paper is to provide a complete picture of the concept of specific heat and its practical applications, helping readers to understand how different substances react to changes in temperature and to make informed choices in the design and optimization of thermal and energy systems.
Knowledge of the specific heat of substances is essential for a number of scientific and engineering disciplines, contributing to technological progress and energy efficiency.
Specific heat substances
Specific heat substances
The amount of energy that a hot body transfers to a colder one depends not only on the difference between the two temperatures, but also on the mass of the hotter body. In fact, the energy that the soleplate of an iron can transfer is much greater than that which a pin can transfer at the same temperature. Heat is therefore an extensive quantity of matter. Its unit of measurement is the same as energy, which in SI is the joule. The effect caused on a body by a certain amount of heat depends on the nature of the body.
When, for example, we heat 1 kg of water and 1 kg of iron with equal quantities of heat, we record different final temperatures. It follows that, as the nature of the body varies, the quantity of heat necessary to increase the temperature of 1 kg of mass by 1 K differs. This amount of heat is called specific heat.
Specific heat is the amount of energy absorbed (or released) by 1 kg of material that causes a 1 K increase (or decrease) in temperature.
Measure unit
The unit of measurement of specific heat, which is a derived quantity, is J/kg K in the SI. The units J/g K (or °C), and cal/g °C are also often used, for water it is , for example, 4186 J/kg K, which corresponds to 4,186 J/g °C and 1 cal/g °C
The specific heat of water is very high: that is, a lot of energy is needed to obtain small increases in temperature. The specific heat of copper, on the other hand, is quite small, so small amounts of energy cause large increases in temperature. The instrument that allows to measure the quantity of energy transferred is the calorimeter: the quantity of energy transferred is the calorimeter: with this device the changes in temperature that the transfer of energy causes on a certain body, usually water, are recorded.
Taking into account the mass, the specific heat and the temperature difference, the energy can be calculated with the following formula:
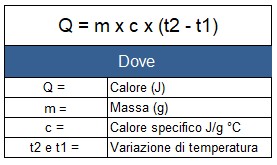
Specific heat substances
The program below is free to use.
To access the reserved version (see below), full page and without advertising, you must be registered.
You can register now by clicking HERE