Specific gravity liquids and solutions
Specific gravity, liquids and solutions.
This paper created by Itieffe, is a document dedicated to exploring in detail the concept of specific gravity when applied to liquids and solutions. This topic is of fundamental importance in various scientific and engineering fields, since understanding the specific gravity of liquids is crucial to a number of practical applications and to the design of fluid transport systems.
In this paper, we will address the following key points:
- Definition of specific gravity: we will explain the concept of specific gravity as the density of a liquid, that is, the amount of mass per unit volume of the liquid itself. We will introduce common terms and units of measurement, such as the kilogram per cubic meter (kg/m³) in the International System (SI).
- Variability of specific gravity: we will see how the specific weight of liquids can vary based on chemical composition, temperature and pressure. This variation is important to consider when working with liquids under different conditions.
The main objective of this paper is to provide a comprehensive information resource for anyone interested in the specific gravity of liquids and solutions. Understanding this concept is critical in many fields, including civil engineering, hydraulics, chemistry, materials science, and the design of fluid transportation and distribution systems. A solid understanding of the specific gravity of liquids and solutions is essential for proper design and management of systems involving these materials.
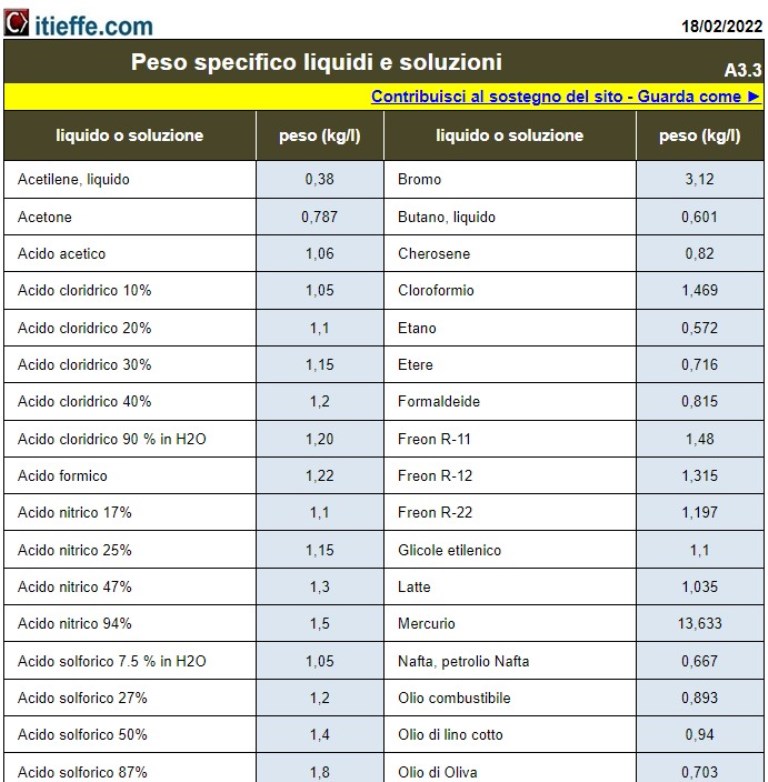
Specific gravity liquids and solutions
“Specific gravity”, also known as density, is a physical quantity that represents the amount of mass of a substance contained in a given unit of volume. In other words, specific gravity measures how much matter is concentrated in a specific space. It is usually expressed in units of mass per unit of volume, such as kilograms per cubic meter (kg/m³) in the International System (SI).
The general formula for calculating specific gravity is:
Specific Weight=Mass/Volume.
Where:
- “Mass” represents the amount of matter contained in the object or substance, usually measured in kilograms (kg).
- “Volume” indicates the space occupied by that mass, usually measured in cubic meters (m³).
Specific gravity can vary greatly between different substances and materials. For example, the specific weight of water is approximately 1000 kg/m³ at room temperature. This means that one cubic meter of water has a mass of 1000 kilograms. Denser materials, such as iron, will have a much higher specific gravity, while less dense materials, such as air, will have a much lower specific gravity.
Knowledge of specific gravity is fundamental in several scientific and engineering disciplines, as it affects various aspects, including the load-bearing capacity of structures and the choice of materials in specific applications.
Specific gravity liquids and solutions
Other free programs of the same kind offered by itieffe ▼
Specific gravity liquids and solutions
The program below is free to use.
To access the reserved version (see below), full page and without advertising, you must be registered.
You can register now by clicking HERE
